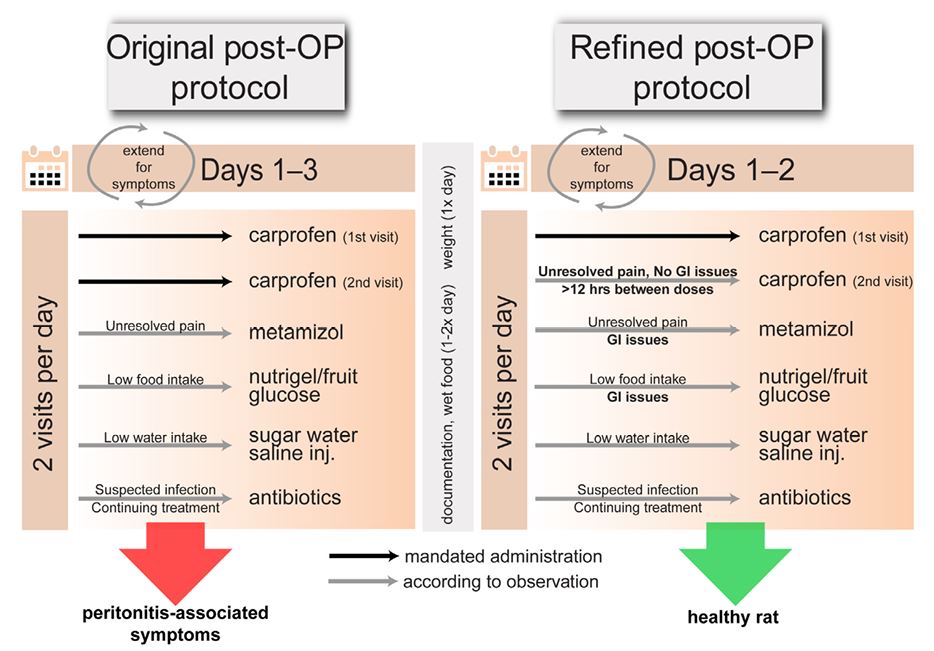
Abstract
Effective pain management in laboratory animals is crucial for both animal welfare and the reliability of scientific research. We retrospectively examined the effects of carprofen as post-operative analgesia in Sprague Dawley rats following stereotactic surgery. Our data indicate that administering carprofen twice daily (5 mg/kg), as currently recommended by Die Gesellschaft für Versuchstierkunde/Society for Laboratory Animal Science (GV-SOLAS), led to adverse effects such as reduced food and water intake, disrupted fecal excretion, and abdominal bloating consistent with peritonitis. Continued administration exacerbated these symptoms, with post-mortem findings of intestinal obstructions and ulcers. However, when the frequency was reduced to once daily, such adverse symptoms were not observed. These results are based on incidental data collected from various neuroscientific experiments, resulting in small and uneven sample groups across various experimental cohorts. The inherent imbalances in these groups present challenges for statistical interpretation. While the findings suggest that less frequent carprofen use may reduce adverse effects, the surgical interventions and concurrent use of other drugs in these experiments likely exacerbated these outcomes. Further investigation into the interactions between carprofen, surgical stress, and other perioperative factors is needed to refine analgesia protocols in laboratory animals. Despite these limitations, these observations contribute to understanding analgesia protocols and may assist in improving animal welfare practices.