Highlights
Abstract
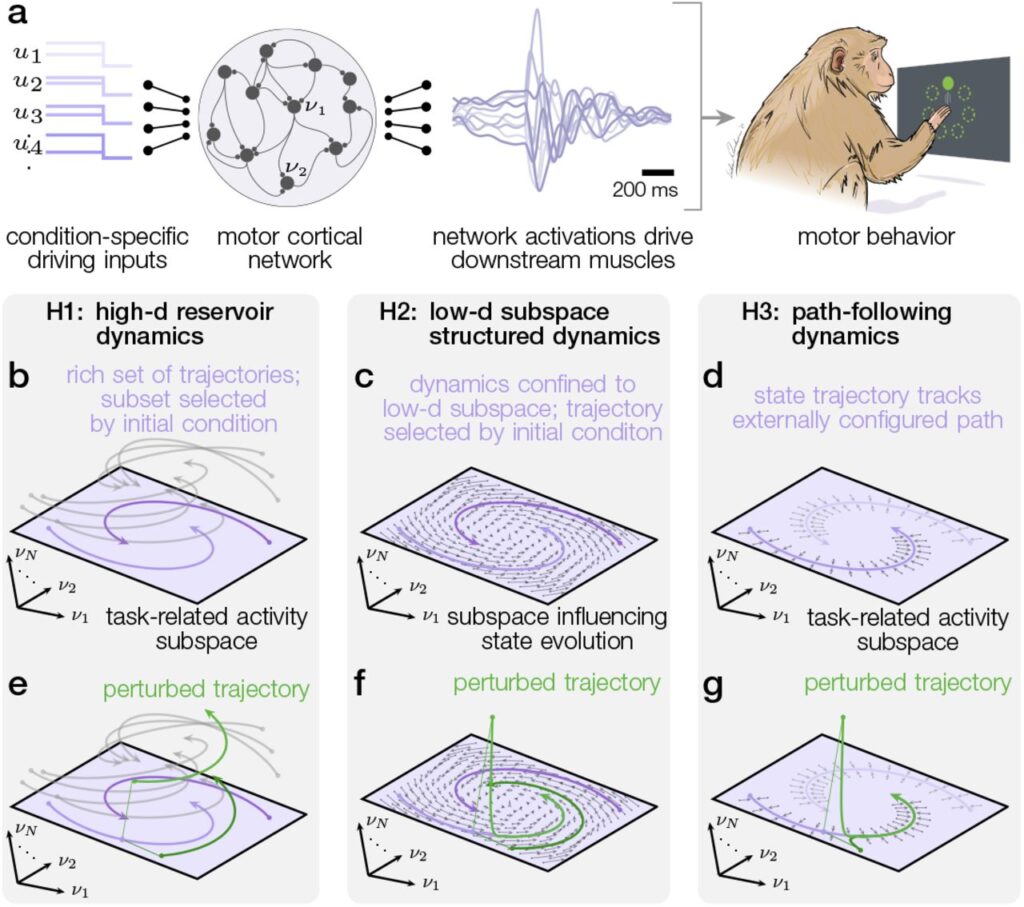
The rich repertoire of skilled mammalian behavior is the product of neural circuits that generate robust and flexible patterns of activity distributed across populations of neurons. Decades of associative studies have linked many behaviors to specific patterns of population activity, but association alone cannot reveal the dynamical mechanisms that shape those patterns. Are local neural circuits high-dimensional dynamical reservoirs able to generate arbitrary superpositions of patterns with appropriate excitation? Or might circuit dynamics be shaped in response to behavioral context so as to generate only the low-dimensional patterns needed for the task at hand? Here, we address these questions within primate motor cortex by delivering optogenetic and electrical microstimulation perturbations during reaching behavior. We develop a novel analytic approach that relates measured activity to theoretically tractable, dynamical models of excitatory and inhibitory neurons. This computational model captures the dynamical effects of these perturbations and demonstrates that motor cortical activity during reaching is shaped by a self-contained, low-dimensional dynamical system. The subspace containing task-relevant dynamics proves to be oriented so as to be robust to strong non-normal amplification within cortical circuits. This task dynamics space exhibits a privileged causal relationship with behavior, in that stimulation in motor cortex perturb reach kinematics only to the extent that it alters neural states within this subspace. Our results resolve long-standing questions about the dynamical structure of cortical activity associated with movement, and illuminate the dynamical perturbation experiments needed to understand how neural circuits throughout the brain generate complex behavior.
